A3G – AUTOMATED ANDERSEN CASCADE IMPACTOR
A3G CONFIGURATIONS
-

pMDI CONFIGURATION
-

NASAL CONFIGURATION
-

DPI CONFIGURATION
-
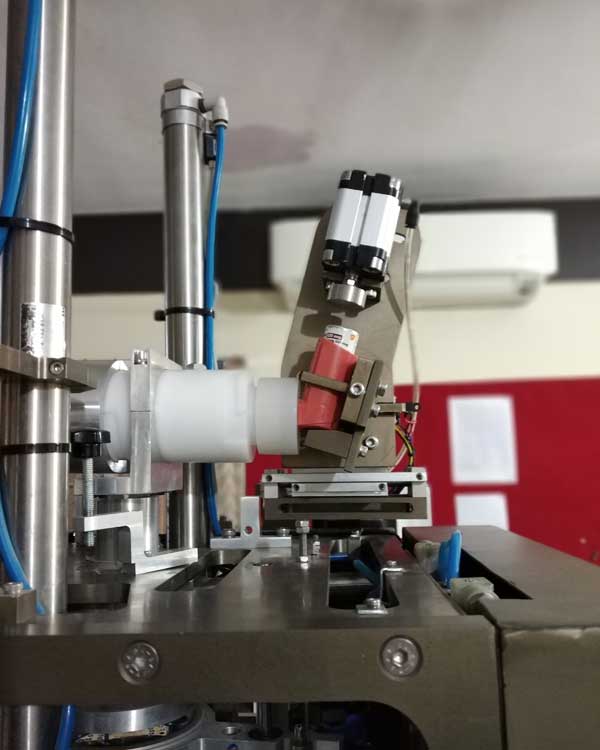
DUSA CONFIGURATION
Advantages of A3G
- Versatile machine. Can be user configured for Oral or Nasal APSD test, and for 30/60/90 LPM in a matter of minutes. Can Dose in ACI Column or in DUSA Tube.
- In the the Nasal Configuration, it can count the number of Nasal Sprays injected into the Glass Expansion Chamber
- Can be configured to do APSD for pMDI or DPI at 30/60/90 lpm
- Nasal APSD test can be done using 2L or 5L Glass Expansion Chamber
- Integrated inhaler shaker and dose injector
- Produces very high quality APSD data. Tests with Fluticasone and Beclomethasone have shown RSD under 2%. With high mass balance(97%+), the data can be used to report DDU in addition to reporting APSD
- High throughput—can do sample prep for a APSD every 20 minutes, including washing & drying. Automated concurrent dose collection from individual ACI stages and impaction plates
- Separate automated dose collection from pre-separator and Induction Port
- Easy migration of existing ACI methods to A3G, as ACI within the A3G remains unchanged, so very easy to bridge from ACI to A3G
- Automated flow rate set up with in-built mass flow controller
- Automated & programmable solvent injection and operator programmable dose dissolution
- Reduces Solvent Consumption, and hence reduces both solvent and solvent disposal cost—environmentally friendly!
- Lifelong filter–One micron 316 Stainless Steel woven wire mesh filter with zero bypass air flow
- CFR21/11 compliant windows based software and distributed control system. Logs and methods are stored in a PL/SQL database that can be integrated with a LIMS system.
- Methods created under version control w/ audit trail of method changes
- Access control — operator login to run methods. Supervisors have full access
- Validation documents available
HIGH PRECISION DATA
Recovery of Beclomethasone Dipropionate (n=3)
| Sample | Area (mV.s) | ||||
| Trial 1 | Trial 2 | Trial 3 | Mean | %RSD | |
| Mouth Piece + Induction Port + Entrance Cone | 633916 | 667525 | 694897 | 665446 | 4.59 |
| IP 1+S0 | 106631 | 92958 | 100562 | 100050 | 6.85 |
| IP 2+S1 | 21901 | 23074 | 24430 | 23135 | 5.47 |
| IP 3+S2 | 26202 | 25218 | 24759 | 25393 | 2.90 |
| IP 4+S3 | 128433 | 125085 | 125093 | 126203 | 1.53 |
| IP 5+S4 | 473662 | 455849 | 465866 | 465125 | 1.92 |
| IP 6+S5 | 695762 | 681555 | 693435 | 690250 | 1.10 |
| IP 7+S6 | 310304 | 306707 | 309955 | 308988 | 0.64 |
| IP 8 +S7 | 151644 | 156621 | 155060 | 154441 | 1.65 |
IP =Impaction Plate. IP1 is below Stage 0, IP2 is below Stage 1, and in this manner, finally, IP8 is below Stage 7
Recovery of Fluticasone Propionate using GSK Flovent. The sample prep was done using the A3G and the Chromatography was done by ZYDUS PHARMACEUTICALS.
| (GSK Flovent pMDI) Fluticasone Propionate ACI | ||||||||||
| RUN 1 | RUN 2 | RUN 3 | RUN 4 | RUN 5 | RUN 6 | RUN 7 | RUN 8 | |||
| Sr. No. | Sample name | % LC-220 mcg | % LC-220 mcg | % LC-220 mcg | % LC-220 mcg | % LC-220 mcg | % LC-220 mcg | % LC-220 mcg | % LC-220 mcg | %RSD |
| 1 | Mouthpiece Adaptor | 5.81 | 5.68 | 5.29 | 5.69 | 5.69 | 5.85 | 5.45 | 5.64 | 3.27 |
| 2 | Induction Port | 48.24 | 45.32 | 45.87 | 45.56 | 47.96 | 44.13 | 41.74 | 43.02 | 4.97 |
| 3 | Cone | 0.12 | 0.13 | 0.13 | 0.12 | 0.14 | 0.1 | 0.1 | 0.12 | 11.79 |
| 4 | Impaction Plate-1 + Stage 0 | 3.03 | 2.83 | 2.88 | 2.76 | 2.9 | 3.06 | 2.69 | 3.45 | 8.04 |
| 5 | Impaction Plate-2 + Stage 1 | 1.86 | 3.3 | 3.39 | 3.44 | 3.7 | 3.45 | 3.52 | 3.75 | 18.23 |
| 6 | Impaction Plate-3 + Stage 2 | 5.05 | 5.11 | 5.31 | 5.55 | 5.92 | 5.63 | 5.62 | 5.82 | 5.76 |
| 7 | Impaction Plate-4 + Stage 3 | 14.3 | 14.84 | 15.32 | 16.25 | 16.22 | 15.27 | 15.41 | 15.92 | 4.38 |
| 8 | Impaction Plate-5 + Stage 4 | 15.05 | 14.95 | 15.5 | 16.32 | 15.86 | 15.39 | 15.49 | 15.78 | 2.86 |
| 9 | Impaction Plate-6 + Stage 5 | 5.53 | 5.29 | 5.4 | 5.75 | 5.71 | 5.5 | 5.57 | 5.5 | 2.72 |
| 10 | Impaction Plate-7 + Stage 6 | 0.7 | 0.67 | 0.68 | 0.72 | 0.72 | 0.72 | 0.68 | 0.72 | 3.09 |
| 11 | Impaction Plate-F + Stage 7 | 0.2 | 0.28 | 0.2 | 0.25 | 0.28 | 0.27 | 0.24 | 0.24 | 13.09 |
| 12 | Stage-F | 0.17 | 0.22 | 0.24 | 0.25 | 0.22 | 0.2 | 0.19 | 0.21 | 12.26 |
| Mass Balance | 100.06 | 98.62 | 100.21 | 102.66 | 105.32 | 99.57 | 96.7 | 100.17 | 2.58 | |
Comparison of A3G technology Vs. NGI
- value1
- value2
- A3G
- NGI
- 1
- Flight path of the aerosol particles
- A3G: Same as in the ACI
- NGI: 2x -It has to be cooled before each use for pMDIs
- 2
- Drug extraction
- A3G: Not an issue- greater than 98 % Drug recovery
- NGI: Gentle rocking of the cups after adding solvent not sufficient to recover the entire drug from the excipient matrix.Energetic agitation leads to spillage
- 3
- Limitations on amount of solvent per cup
- A3G: Can be customized for desired volume of solvent
- NGI: The rocking motion sloshes the solvent around in the cup – limits the max amount to ~20 mL to avoid spill-over. Difficult to test inhalers which deliver large fractions of the dose per actuation.
- 4
- Evaporation of solvents during drug extraction
- A3G: Sealed system -solvent evaporation is not an issue
- NGI:Open system limits it to using low volatility solvents – can lead to high data variability
- 5
- Cleanup of cups after sample extraction
- A3G: Automated
- NGI:Manual clean up- automated clean up not possible
- 6
- Energetic agitation of cups to dissolve the drug
- A3G: Possible – user programmable
- NGI:Not possible -the solvent sloshes out of the cup
- 7
- Productivity
- A3G: 40-60 APSDs/day
- NGI:Four to ten APSDs/day
- 8
- Mass Balance
- A3G: >98% (proof of concept device)
- NGI:80%Cleaning difficult Crossover/contamination , high data variability poor reliability
- 9
- Impactor Plate/cup handling
- A3G: No manual intervention
- NGI:Manual cup handling increases chances of analyst error, and limiting throughput
- 10
- Automated Dosing of Cascade Impactor
- A3G:A Robot does the dosing in A3G- reduces dosing related errors
- NGI:Automated dosing not possible- leads to greater variability in the data
- 11
- Plate/cup Saturation
- A3G:Even distribution of particles throughout
- NGI:NGI has fewer jets per stage therefore concentrating impacting particles on a smaller surface area.
- 12
- Air Flow
- A3G:Up to 90 liters per minute with minus stages
- NGI:Up to 100 liters per minute
- 13
- Plate Coating for DPIs
- A3G:No coating required
- NGI:Requires cups/plates be coated
- 14
- CFR21 Part 11 compliance
- A3G:Yes
- NGI: Yes
Cascade Impactors
There are several cascade impactors available on the market for measuring particle size distribution from inhaled drugs or an air sample. Most popular are the Andersen Cascade Impactor (ACI) and the Next Generation Impactor (NGI).
Andersen Cascade Impactor (ACI)

The manual ACI process greatly suffers from low productivity, typically two to four dose determinations per day and from operator induced data variability. Hence it takes years to generate the data and get regulatory approval from the FDA.
After drug injection, the 22 components of the ACI must be disassembled in a clean room, and each of its components washed, weighed, dried and samples are collected in duplicate from each disc and HPLC technique is used to determine the drug content. Assuming mass balance, the particle size is deduced from the layer it was collected and the particle size distribution for the dose is drawn up. The data from each process step has to be accurately recorded. Thus the process is slow there are many opportunities for errors when this process is manually executed.In order to understand the limitations of the technologies, we need to understand the technologies.

The Next Generation Impactor or the NGI

The picture shows the Next Generation Impactor. To increase productivity the number of individual parts has been reduced considerably. All of the cups shown in the bottom of the picture are integrated into a tray thus the operator has to handle a tray at a time. However the scope for automation is limited to solvent dispensing and sample collection. The washing is still all manual. The air passages are integrated into the body of the impactor (visible as large holes in the picture). They are difficult to clean and lead to drug accumulation and cross-over data errors. Further, the flight path of the aerosol particle is twice as long as in the ACI. This long path leads to droplet evaporation in case of the pMDI, and the errors tend to be biased towards fine particle fraction. To prevent droplet evaporation, scientists have to freeze/cool the NGI prior to use, making its productivity lower than claimed and the process expensive.
